1. Complex Trial Designs
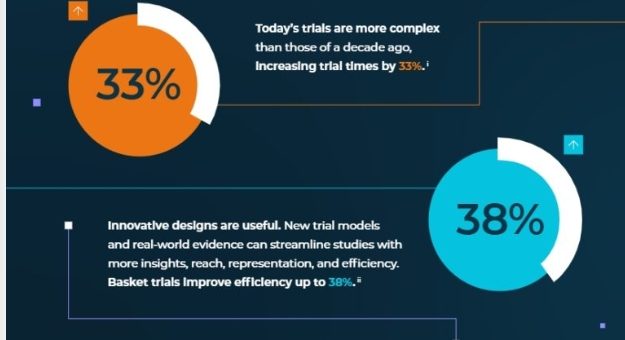
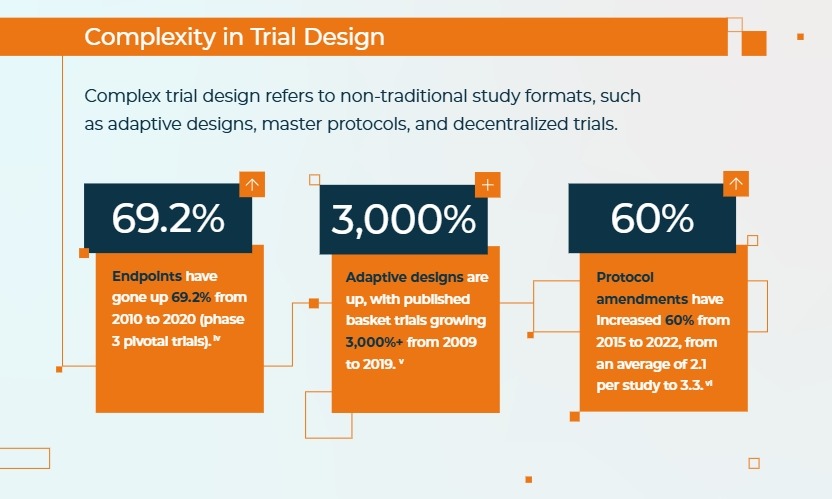
Trial designs have evolved over the past decade, incorporating innovative models like adaptive designs, master protocols, and decentralized approaches. While these non-traditional formats offer the potential for greater flexibility, efficiency, and inclusion, they also introduce significant challenges.
- Adaptive Designs allow for modifications to the trial while it’s ongoing, improving efficiency and relevance.
- Master Protocols (like basket and umbrella trials) can streamline studies, but they require extra time to implement. For example, completing a master protocol trial can take up to 1.9 years longer than traditional methods.
- Decentralized Trials increase patient reach and convenience, yet may complicate data collection and site coordination.
Despite these challenges, innovative trial designs can reduce the overall trial timeline and increase the diversity of data, but their complexity demands careful planning and management.
2. Complex Data
The growing volume and diversity of data in clinical trials — including real-world evidence (RWE), patient-reported outcomes, and more — is another key challenge. While these data sources can increase the relevance and scope of clinical studies, they also pose risks:
- Data Overload can lead to inefficiencies if not properly managed, adding to site workloads and potentially derailing timelines.
- Data Integration is a significant hurdle, as different formats and systems can slow down the analysis and reporting of results.
However, with proper tools for managing and analyzing large and diverse datasets, clinical trials can uncover deeper insights, reduce biases, and increase the applicability of findings to a broader patient population.
3. Complex Operations
The operational side of clinical trials — including site management, patient recruitment, regulatory compliance, and overall project management — has become more challenging as trials grow in size and scope. For example:
- Longer timelines due to the complexity of study designs.
- Increased site workloads from managing diverse data sources and adapting to new trial models.
- Budget pressure as additional resources are needed to manage these complexities.
Despite these operational challenges, there are strategies to enhance efficiency and streamline operations, such as leveraging AI for patient recruitment, using decentralized technologies to reduce site burden, and implementing more robust project management systems to track progress and costs in real-time.
This article is posted at eclinicalsol.com and biopharmadive.com

Please fill out the form to access the content






